|
The objective of the International Laboratory “LIME” is to prepare and characterize ionomer materials for electrochemical energy technologies. Aromatic polymers are very attractive given their easy functionalization, low cost and easy recycling. Among the many aromatic polymers, we focus mainly on Poly(ether-ether-ketone) (PEEK), Poly(phenyl-sulfone) (PPSU), Polysulfone (PSU), and Poly(ether-sulfone) (PES).
1. Proton conducting ionomers
Proton conducting ionomers are materials of choice for fuel cells and water electrolysis. The properties can be improved by innovative thermal treatments for annealing and cross-linking (XL-SPEEK) the macromolecules.
2. Cation conducting ionomers
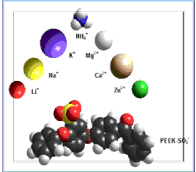
Various cations can be introduced by ion exchange or chemical reaction (e.g. with butyl-Li). The ionomers can be used as separators for rechargeable batteries (in anhydrous state) or for aqueous metal batteries.
3. Hydroxide conducting ionomers
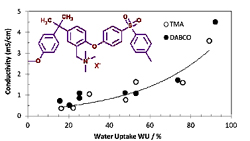
The most common ionomers contain quaternary ammonium groups. The basic operating conditions allow using non-noble electrocatalysts for the oxygen reduction reaction and thus an important cost reduction for alkaline fuel cells and water electrolysers.
4. Anion conducting ionomers
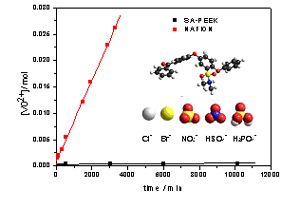
They can be prepared by ion exchange from hydroxide conducting ionomers or by innovative synthesis by reaction of acids with basic groups (e.g. sulfonamides). Such ionomers are especially useful for redox flow batteries, where cation permeability must be minimized.
5. Amphoteric ionomers
Acidic and basic groups coexist in a single macromolecule: the type and value of the ionic conductivity depends on the pH of its environment. This versatility and the low ionic permeability are very useful for technologies requiring low ion permeability.
Ionomer separators for electrochemical energy technologies are produced from the microscale (microbatteries based on TiO2 nanotubes) to the macroscale (redox flow batteries). The characterization techniques include spectroscopies (FTIR, UV and NMR), thermogravimetry, mechanical tests, permeability measurements, impedance spectroscopy and electrochemical measurements, such as cyclovoltammetry and galvanostatic polarization.
Publications
-
G. Barbieri, A. Brunetti, M. L. Di Vona, E. Sgreccia, P. Knauth, H. Y. Hou, R. Hempelmann, F. Arena, L. D. Beretta, B. Bauer, M. Schuster, J. O. Ossó, L. F. Vega, LoLiPEM: Long life proton exchange membrane fuel cells, International Journal of Hydrogen Energy, 41, 1921-1934 (2016).
-
L. Pasquini, M. L. Di Vona, P. Knauth, Effects of anion substitution on hydration, ionic conductivity and mechanical properties of anion-exchange membranes, New Journal of Chemistry, 40, 3671-3676 (2016).
-
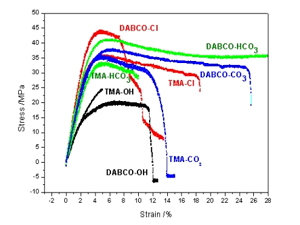
R. Narducci, J.-F. Chailan, A. Fahs, L. Pasquini, M. L. Di Vona, P. Knauth, Mechanical Properties of Anion Exchange Membranes by Combination of Tensile Stress-Strain Tests and Dynamic Mechanical Analysis, Journal of Polymer Science Part B-Polymer Physics, 54, 1180-1187 (2016).
-
R. Narducci, L. Pasquini, J.-F. Chailan, P. Knauth, M. L. Di Vona, Low-Permeability Poly(ether Ether Ketone)-Based Ampholytic Membranes, ChemPlusChem, 81, 550-556 (2016).
-
V. Morizur, M. Braglia, S. Olivero, J.-R. Desmurs, P. Knauth, E. Dunach, New aromatic polymer electrolytes for application in lithium metal batteries, New Journal of Chemistry, 40, 7840-7845 (2016).
-
M. Braglia, I. V. Ferrari, F. Vacandio, P. Knauth, M. L. Di Vona, Anodic Electropolymerization of Sulfonated Poly(phenyl ether): Study of Precursor Isomers and Polymer Growth, ChemistrySelect 1, 3114-3119 (2016).
-
P. Knauth, L. Pasquini, M. L. Di Vona, Comparative study of the cation permeability of protonic, anionic and ampholytic membranes, Solid State Ionics, 300, 97-105 (2017).
-
M. L. Di Vona, M. Casciola, A. Donnadio, M. Nocchetti, L. Pasquini, R. Narducci, P. Knauth, Anionic conducting composite membranes based on aromatic polymer and layered double hydroxides, Int. J. Hydrogen Energy, 42, 3197-3205 (2017).
-
I. V. Ferrari, M. Braglia, T. Djenizian, P. Knauth, M. L. Di Vona, Electrochemically engineered single Li-ion conducting solid polymer electrolyte on titania nanotubes for microbatteries, J. Power Sources, 353, 95-103 (2017).
-
M. Braglia, I. V. Ferrari, T. Djenizian, S. Kaciulis, P. Soltani, M. L. Di Vona, P. Knauth, Bottom-up electrochemical deposition of poly(styrene sulfonate) on nano-architectured electrodes, ACS Applied Materials and Interfaces, 9, 22902-22910 (2017).
-
Z. Derbali, A. Fahs, J.-F. Chailan, I. V. Ferrari, M. L. Di Vona, P. Knauth, Composite anion exchange membranes with functionalized hydrophilic or hydrophobic titanium dioxide, Int. J. Hydrogen Energy, 42, 19178-19189 (2017).
-
R. Pizzoferrato, E. Ciotta, I. V. Ferrari, M. Braglia, P. G. Medaglia, A. Mattoccia, L. Di Giamberardino, M. Richetta, P. Knauth, M. L. Di Vona, Ionic conductivity of Zn–Al layered double hydroxide films grown on aluminium substrate, Solid State Ionics, 314, 30-35 (2018).
-
M. Braglia, I.V. Ferrari, L. Pasquini, T. Djenizian, M. Sette, M.L. Di Vona, P. Knauth Electrochemical synthesis of thin, dense, and conformal anion exchange membranes with quaternary ammonium groups, Electrochimica Acta, 265, 78-88 (2018)
-
P. Knauth, M. L. Di Vona, Electrochemical Synthesis of Conformal, Thin and Dense Ionomer Separators for Energy Storage and Conversion Devices, Adv. Mater. Lett., 9, 855-860 (2018).
-
R. Narducci, P. Knauth, J.-F. Chailan, M. L. Di Vona, How to improve Nafion with tailor-made annealing, RSC Advances, 8, 27268-27274 (2018).
-
O. Ruzimuradov, M. Braglia, F. Vacandio, P. Knauth, A humidity-sensitive nanocomposite solid ion conductor: sulfonated poly(ether ether ketone) in nanotubular TiO2 or ZrO2 matrix, Journal of Solid State Electrochemistry, 22, 3255-3260 (2018).
-
R. Pizzoferrato, E. Ciotta, I. V. Ferrari, R. Narducci, L. Pasquini, A. Varone, M. Richetta, S. Antonaroli, M. Braglia, P. Knauth, M. L. Di Vona, Layered Double Hydroxides Containing an Ionic Liquid: Ionic Conductivity and Use in Composite Anion Exchange Membranes, ChemElectroChem, 5, 2781-2788 (2018).
-
L. Pasquini, O. Wacrenier, M. L. Di Vona, P. Knauth, Hydration and Ionic Conductivity of Model Cation and Anion-Conducting Ionomers in Buffer Solutions (Phosphate, Acetate, Citrate), J. Phys. Chem. B, 122, 12009-12016 (2018).
-
E. Sgreccia, L. Pasquini, G. Ercolani, P. Knauth, M. L. Di Vona, Stimuli-responsive amphoteric ion exchange polymers bearing carboxylic and amine groups grafted to a cross-linkable silica network, Eur. Polymer J., 112, 255-262 (2019)..
-
R. Narducci, G. Ercolani, R. A. Becerra-Arciniegas, L. Pasquini, P. Knauth, M. L. Di Vona, "Intrinsic" Anion Exchange Polymers through the Dissociation of Strong Basic Groups: PPO with Grafted Bicyclic Guanidines, Membranes, 9, 57 (2019).
-
R. Narducci, E. Sgreccia, G. Ercolani, M. Sette, S. Antonaroli, L. Pasquini, P. Knauth, M. L. Di Vona, Influence of the position of ionic groups in amphoteric polyelectrolytes on hydration and ionic conduction: Side chain vs main chain, Eur. Polymer J., 119, 45-51 (2019).
-
R. Giancola, R.-A. Becerra-Arciniegas, A. Fahs, J.-F. Chailan, M. L. Di Vona, P. Knauth, R. Narducci, Study of annealed Aquivion® ionomers with the INCA method, Membranes, 9, 134 (2019).
-
R.-A. Becerra-Arciniegas, R. Narducci, G. Ercolani, S. Antonaroli, L. Pasquini, P. Knauth, M. L. Di Vona, Alkaline stability of model anion exchange membranes based on poly(phenylene oxide) (PPO) with grafted quaternary ammonium groups: Influence of the functionalization route, Polymer, 185, 121931 (2019).
-
R.-A. Becerra-Arciniegas, R. Narducci, G. Ercolani, E. Sgreccia, L. Pasquini, M. L. Di Vona, P. Knauth, Model Long Side-Chain PPO-Based Anion Exchange Ionomers: Properties and Alkaline Stability, J. Phys. Chem. C, 124, 1309-1316 (2020).
-
L. Pasquini, R.-A. Becerra-Arciniegas, R. Narducci, E. Sgreccia, V. Gressel, M. L. Di Vona, P. Knauth, Properties and Alkaline Stability of Composite Anion Conducting Ionomers Based on Poly(phenylene oxide) Grafted with DABCO and Mg/Al Lamellar Double Hydroxide, CHEMELECTROCHEM, 7, 2917-2924 (2020).
-
M.L. Di Vona, P. Knauth, Electrochemical synthesis of ion exchange polymers: Comparison between hydroxide and proton conductors, Solid State Ionics, 352, 115370 (2020).
|